An introduction to Fluorinated surfactants.
Surfactants are everywhere in our day to day life; shampoos, dish washing detergents, soap, toothpaste and laundry detergents are just some of the most common. These products use based on hydrocarbon surfactants.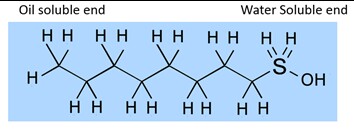
Basically, a hydrocarbon surfactant has an oil soluble section on one end and a water-soluble section on the other end. The oil soluble end is most commonly a linear hydrocarbon chain with from 8 to 18 carbons (most commonly 12-14). The example below has 8 carbons. The carbons are not labelled, they are located where 4 lines intersect.
Fluorinated surfactants come in two common types, perfluorinated (all carbons fully fluorinated) and polyfluorinated, where some hydrogen remain.
Perfluorinated surfactants are more stable, 3M AFFF products (which were removed from the market around 15 years ago) used a high proportion of this type of surfactant, with 8 carbons or more.
Poly fluorinated surfactants will degrade to the shorter perfluorinated chain relatively quickly. In this example below, to a C6 surfactant as only 6 carbons are fully fluorinated. They were commonly manufactured using the telomerisation process (often called fluorotelomers). Many AFFF foams used this type of surfactant. The technology was developed in the 1960’s.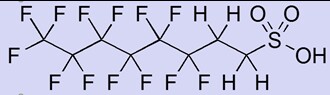
The fluorinated carbon chain is not very soluble in normal hydrocarbons. This is the source of the fuel shedding properties that fluorinated surfactants introduced to fire-fighting foams.
The F-C bond is much more chemically and thermally stable than the H-C bond in hydrocarbon surfactants. This is due to a combination of high bond energy, short bond length and small van der Waals radius. This provides much greater heat resistance for these surfactants, the extremely low surface tension they can achieve and their persistence in the environment.
The extremely low surface tension these surfactants can achieve is the source of the other great advantage of fluorinated fire fighting foams – the aqueous film. The aqueous film floats a more dense layer of water (foam solution) on top of a less dense fuel, when properly formulated. More common hydrocarbon fuels have densities around 80% of water. Without this effect the water (foam solution) would fall through the fuel and not provide any benefit. Also, when properly formulated, this film can act as a vapour barrier.
When AFFF’s were formulated they were formulated so that water drained out reasonably quickly to generate this aqueous film and form a more fluid foam blanket. Fluorine free foams rely on the foam blanket stability for their fire extinguishing, so generally they have much longer drain down times than fluorine containing foams. It is more challenging to keep the foam blanket fluid under these conditions.
Useful References.
https://en.wikipedia.org/wiki/Surfactant
https://en.wikipedia.org/wiki/Organofluorine_chemistry
https://en.wikipedia.org/wiki/Fluorosurfactant